The RNA Biomarker Company
Genialis is the RNA Biomarker company. We envision a world where precision medicine delivers the best possible outcomes for patients, families, and their communities.
We achieve this by working with pharma companies developing the next generation of life-saving oncology drugs, partnering with diagnostics companies building new tests to inform treatment decisions, and collaborating with the world’s leading cancer research institutions.
Introducing Genialis™ krasID,
the only biomarker panel both necessary and sufficient to predict tumor response and clinical benefit to KRAS-targeted therapeutics. We are looking for collaborators across Pharma, Biopharma, Diagnostics, and Research Centers who want to test drive this next-generation patient classifier.
Genialis krasID Early Access Program is accepting inquiries!
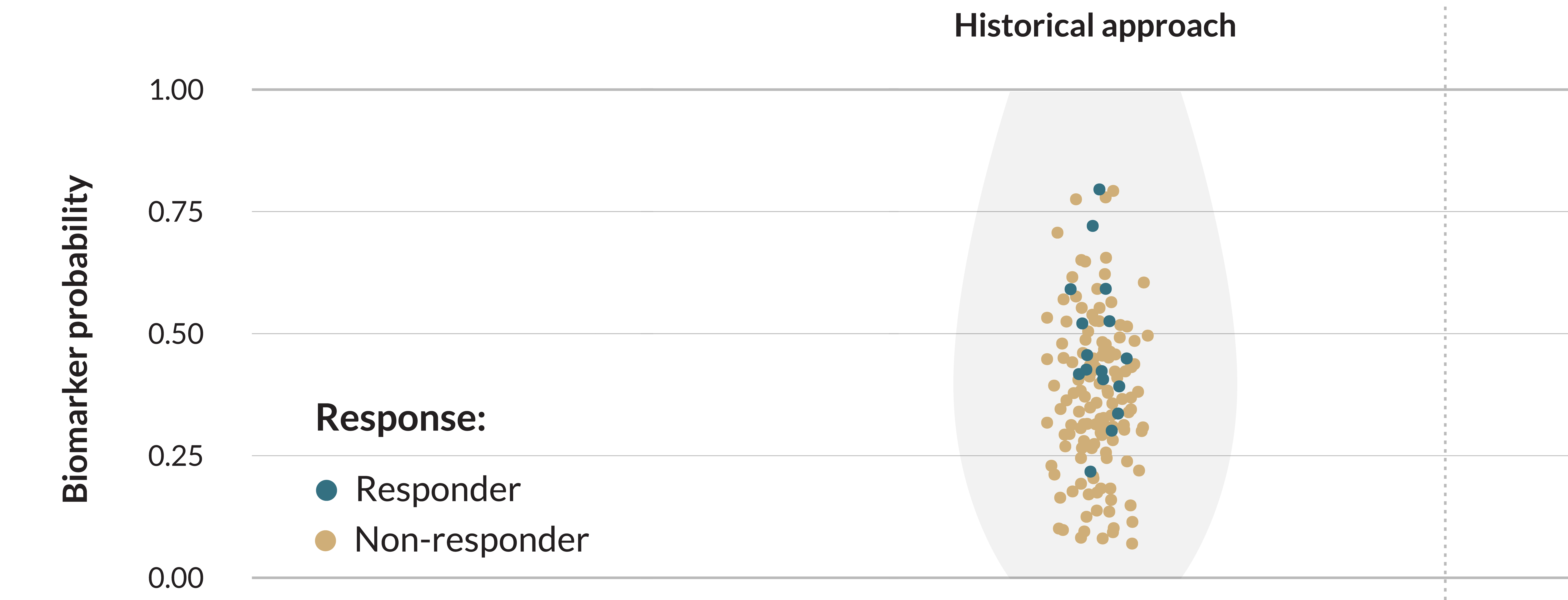
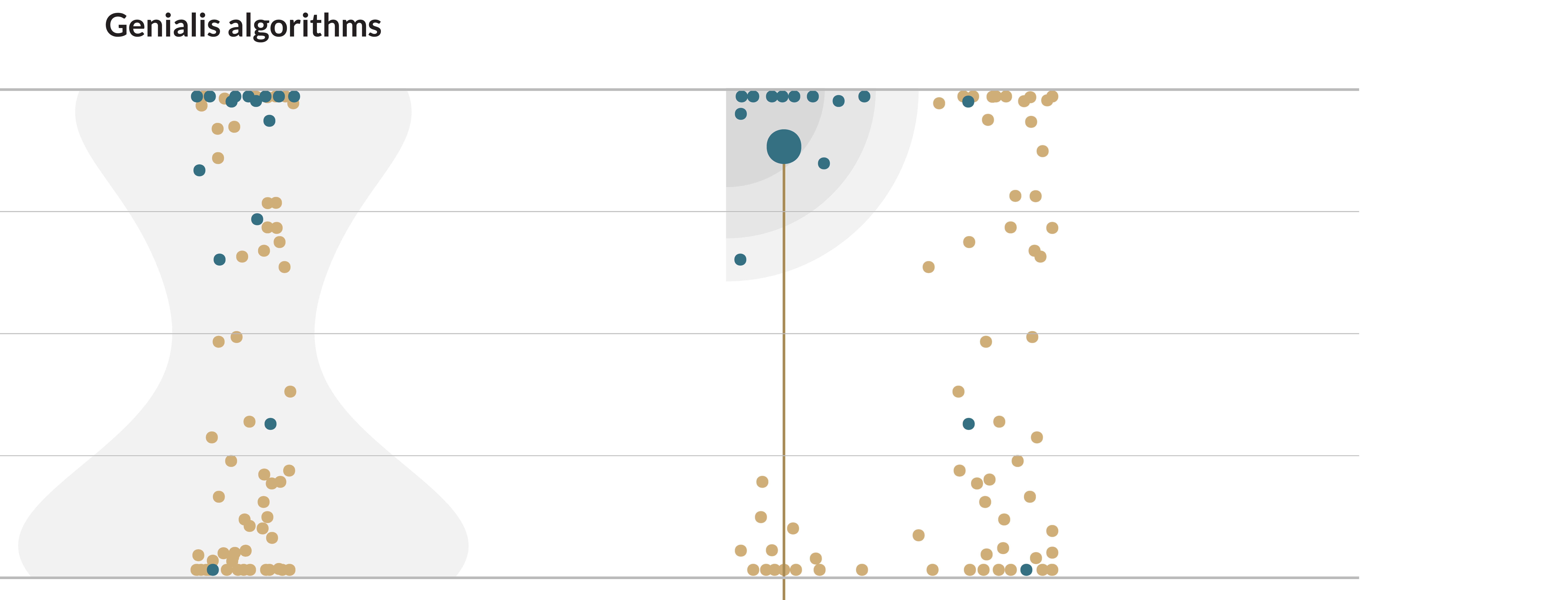
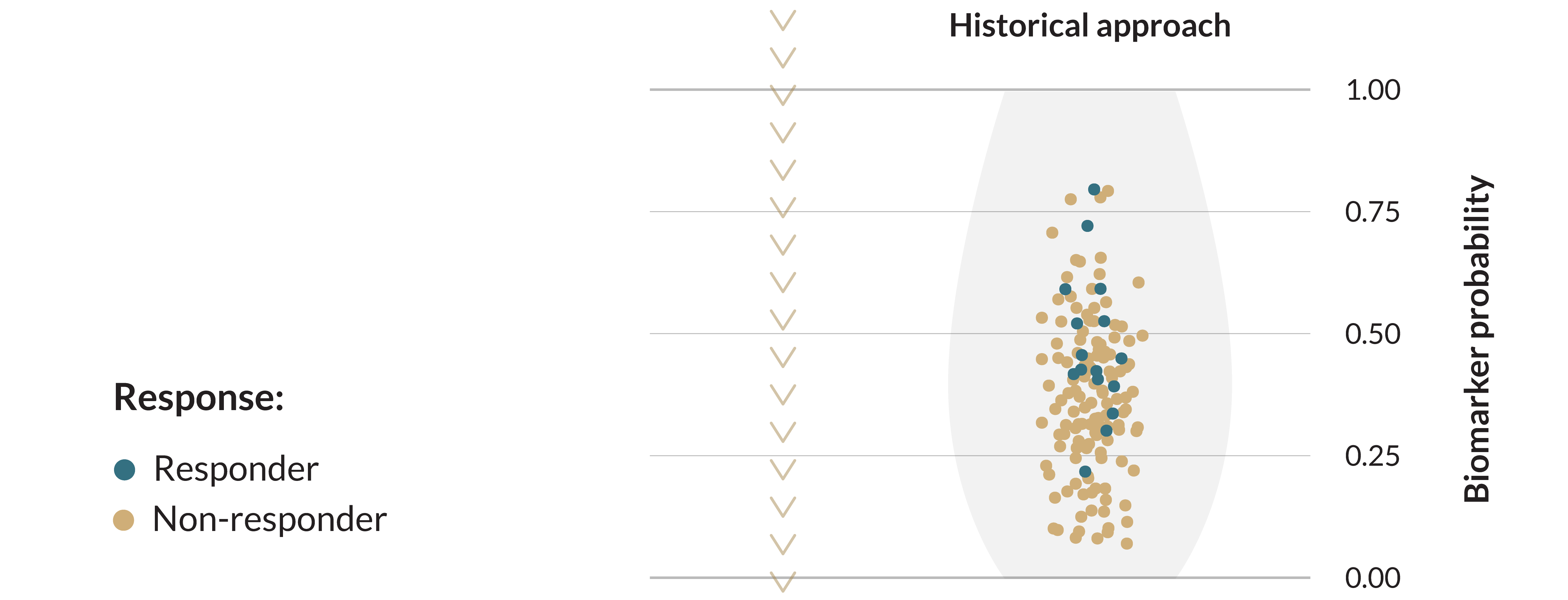
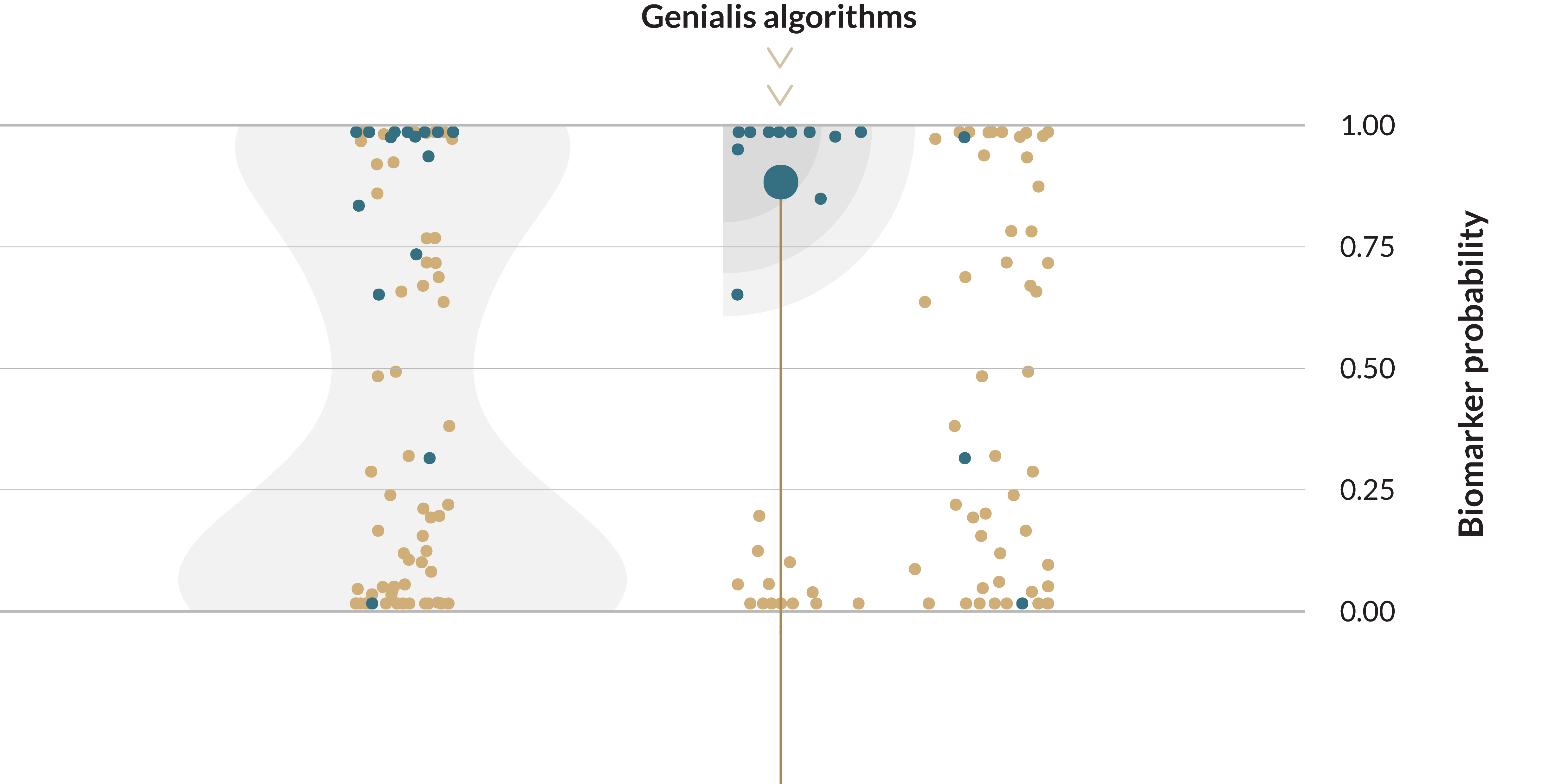
Biomarkers improve response rates
In both real world and clinical trial settings, a greater proportion of patients benefit when selected by biomarkers.
Immune checkpoint inhibitor response rate1
Progression of drug to next clinical phase2



Today’s biomarkers fall short of the industry’s hopes and patients’ expectations in predicting clinical benefit. As of 2020, roughly 27% of cancer patients were eligible for genome-informed therapy, while only 11% of cancer patients showed a clinically beneficial response to such treatment3.
For example, PD-L1 is a valuable prognostic biomarker for overall survival across a variety of cancers4. It has also been approved by the FDA as a companion diagnostic for immune checkpoint therapy. However, a retrospective study of all clinical trials between 2011-2019 prompting FDA approval of immune checkpoint inhibitors identified PD-L1 as a predictive biomarker in only about 30% of cases5.
Featured Partners
“We believe DNA biomarkers can be necessary, but are insufficient. That’s why we build RNA biomarkers, which provide all the information as DNA plus so much more to predict response.”
CEO and Co-founder of Genialis
1 Late-stage gastric cancer cohort, checkpoint inhibitor monotherapy, described in: Strand-Tibbitts K., et al. Society for ImmunoTherapy in Cancer, 2020.
2 Parker JL, et al. Cancer Medicine, 2021.
3 Haslam A, Kim MS, Prasad V. Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006-2020. Ann Oncol. 2021;32(7):926-932. doi:10.1016/j.annonc.2021.04.003
4 Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi:10.1016/S0140-6736(18)32409-7
5 Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. Published 2019 Oct 26. doi:10.1186/s40425-019-0768-9