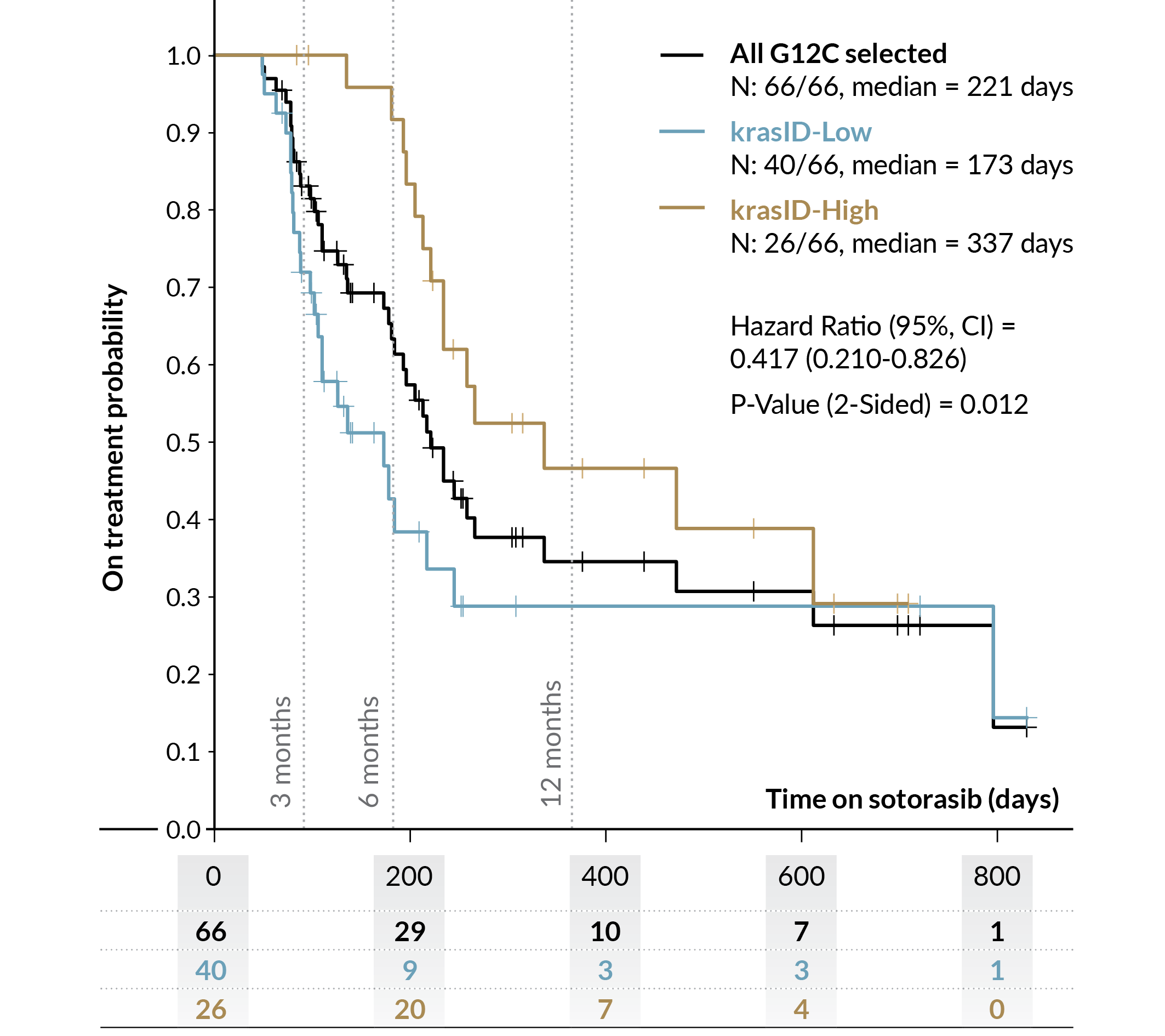
Genialis™ krasID
First-in-class patient classifier predicts response and stratifies clinical benefit of KRAS inhibition
Off-the-shelf or optimized to your compound, Genialis krasID is ready to support your translational and clinical development.
Genialis krasID was developed using the Genialis™ Supermodel and ResponderID™ methodology.
Genialis™ krasID outperforms current Standard of Care Biomarkers, which are limited to mutational status
Historical Approach
Genialis™ krasID Advantage
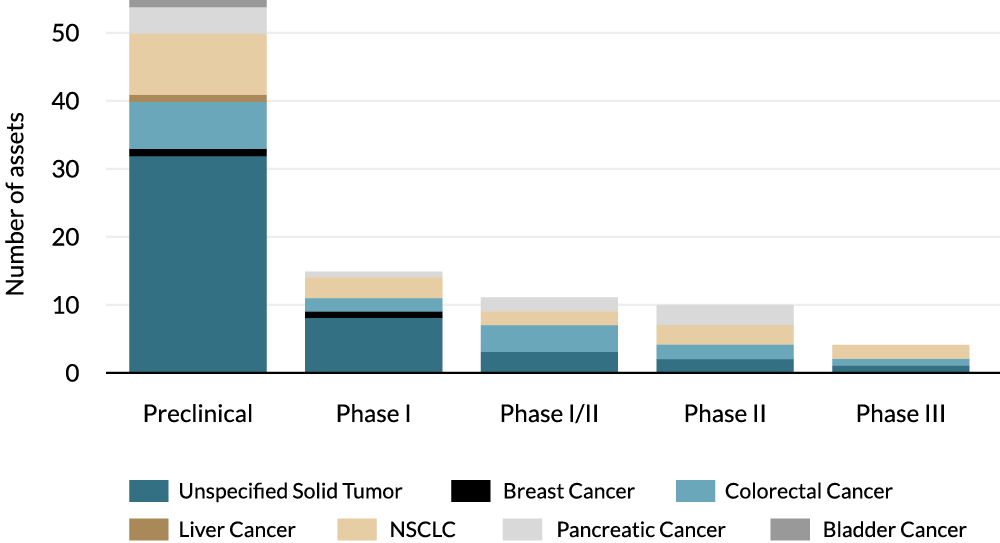
The current KRASi development landscape is crowded; Next-generation biomarkers will provide differentiation
Two drugs currently on market
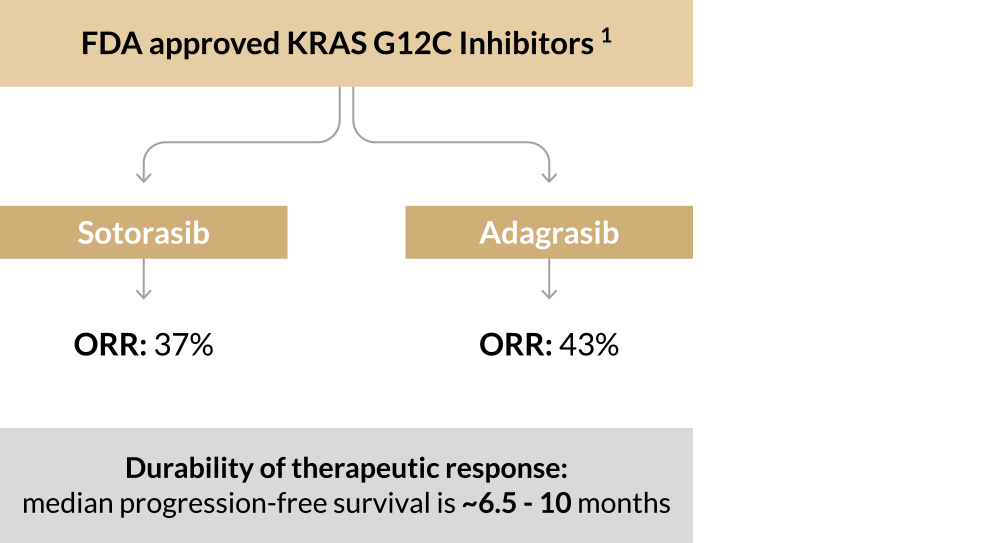
Competition to get to market
>50 companies competing across ~7 different solid tumor indications 2
1 Tian H, Yang Z, He J. Adagrasib: A landmark in the KRASG12C-mutated NSCLC. MedComm (2020). 2022 Nov 25;3(4):e190. doi: 10.1002/mco2.190. PMID: 36448054; PMCID: PMC9697582.
2 Biocentury Inc. “KRAS Pipeline” (2024)