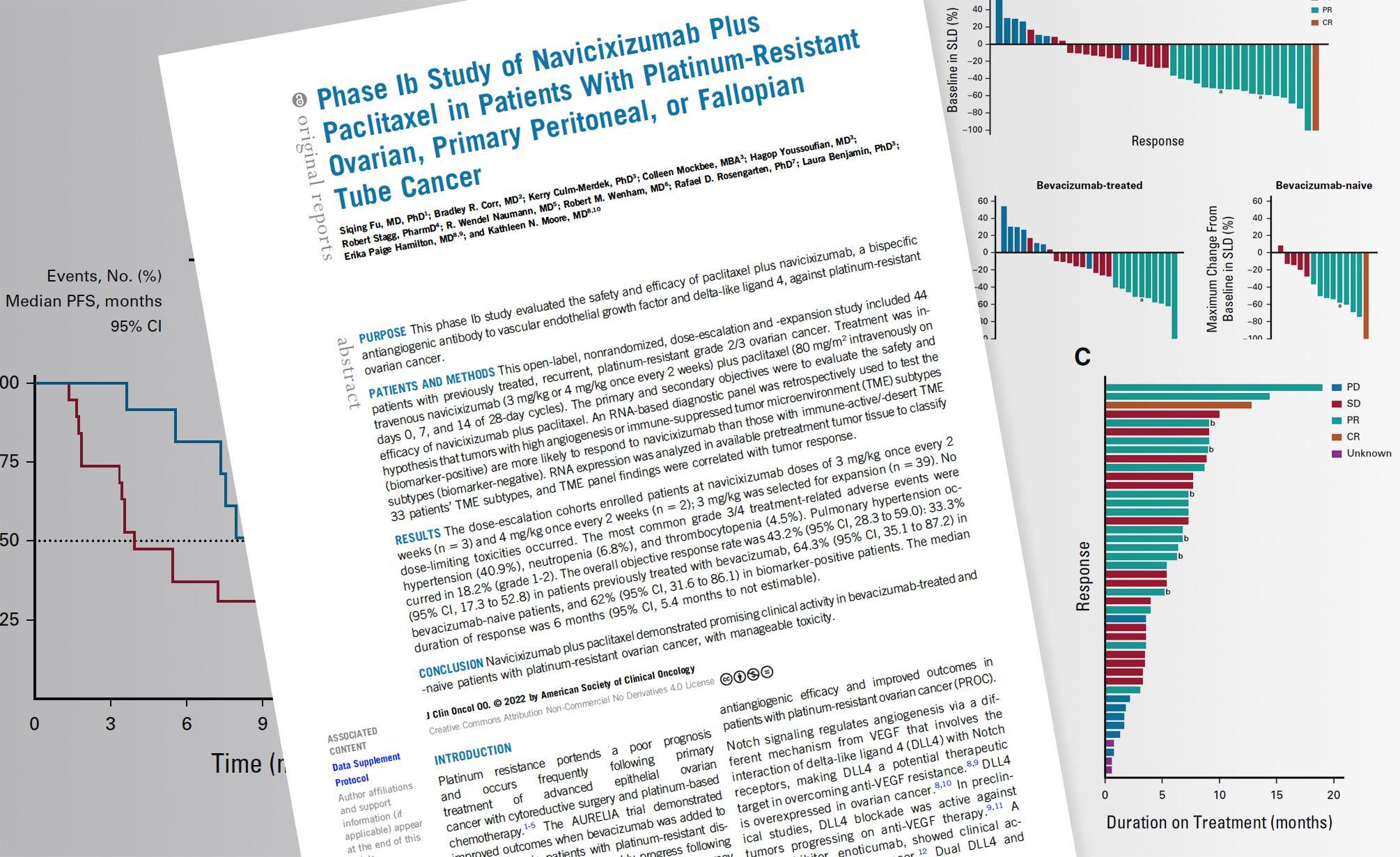
Our CEO Rafael Rosengarten co-authored a new paper on a Phase Ib Study of OncXerna Therapeutics Navicixizumab that demonstrated promising results for the novel bi-specific anti-angiogenic agent with minimal toxicity for patients with platinum-resistant ovarian cancer. The study also showed some compelling results of retrospective analysis with the Xerna TME Panel biomarker as well, which Genialis now uses in collaboration with ResponderID to offer improved targeted therapy selection to its customers.
Share this story, choose your platform!