ResponderID™
A methodology for oncology RNA biomarker discovery at scale
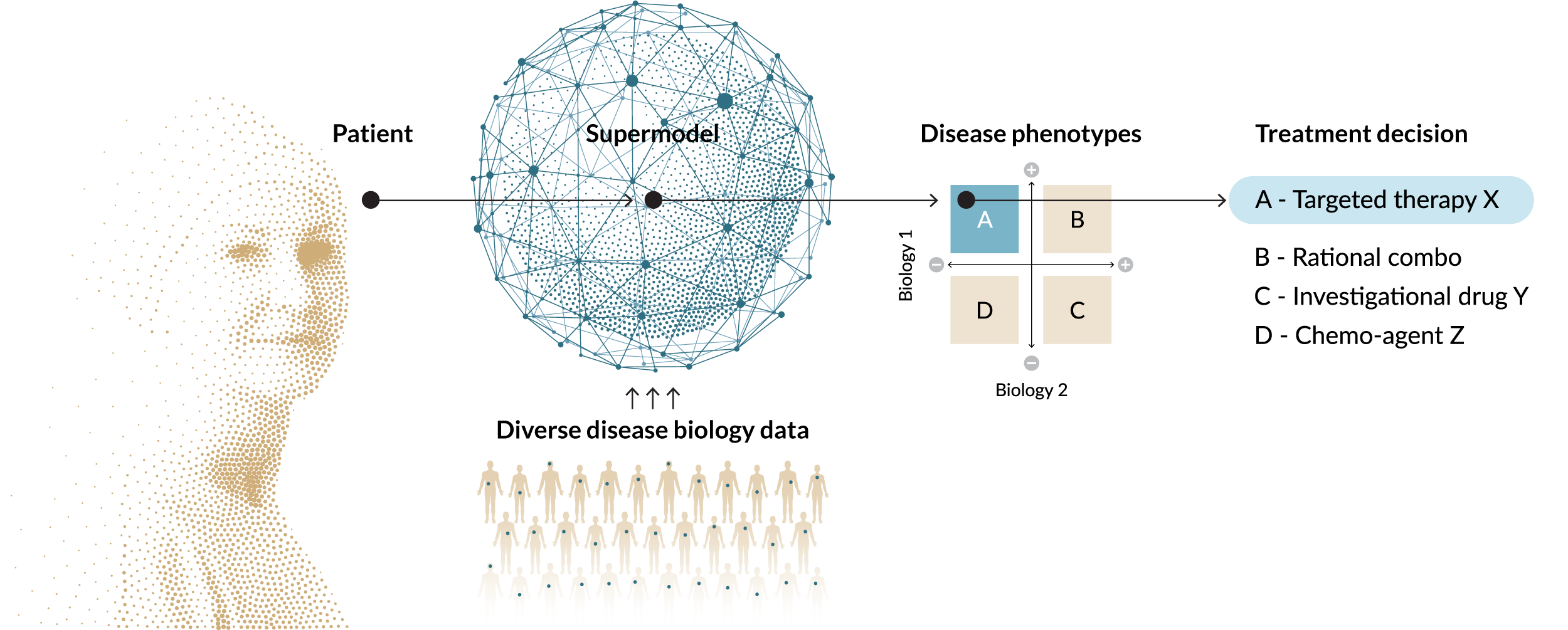
Genialis biomarkers analyze gene expression data with an advanced AI foundation model and machine learning algorithms to predict treatment response and stratify patients.
Over the past two decades of academic research and commercial development, Genialis scientists have honed a novel methodology to ensure its RNA-based biomarkers work across the entire translational and clinical lifecycle of drug development. We have repeatedly shown how biomarkers developed with our methodology perform on patient data from clinical trials and the real world.
"We are so focused on getting the science right, because when we do, it changes lives."
CTO and Co-founder of Genialis
Key Advantages
Why is Genialis ResponderID better than other biomarker approaches?
- Develop, test, and refine biomarkers on preclinical and translational data, even before the first clinical trial results are collected, without sacrificing future accuracy in patient data
- Integrate and extract value from relatively small datasets, e.g. those from clinical trials
- Create models that are generally useful across tissue histologies and accommodate multiple drug MOAs
- Precision medicine / clinical development strategy to maximize the value and impact of the biomarker program
- Testing, tuning and validation to specific compound data to further differentiate the drug from its peers
- Clinical trial assay/ Cdx development with our global network of diagnostic partners
- Accurate and interpretable, due to the information richness of RNA-seq
- Truly representative, with globally diverse training data and a worlds-first RNA foundation model to fully capture complex biology
- Scalable for the life of a drug, from cell lines to trial patients to the real world
