Breast cancer remains a challenging therapeutic area in oncology, especially triple-negative breast cancer (TNBC), which represents 10-20% of breast cancer and has traditionally had very limited treatment options. Immune checkpoint inhibitors (ICI) have emerged as a promising treatment option for TNBC patients. However, identifying those most likely to respond to ICI therapy remains a complex task.
While programmed death-ligand 1 (PD-L1) positivity has long served as the standard for ICI therapy selection in TNBC, Genialis can more precisely predict treatment response with a more intricate examination of the tumor microenvironment (TME).
Understanding TNBC: Currently Used Biomarkers
TNBC historically has had fewer treatment options than other breast cancer subtypes because it is not amenable to hormone therapy or drugs that target HER2. If detected early, surgery and chemotherapy were the only choices, with frequent recurrence of the disease after initial positive response to treatment.
The advent of new targeted therapies has opened additional options for TNBC patients. For example, in stage I-III TNBC patients with BRCA mutation, in certain cases (depending on tumor size and/or lymph node involvement), a PARP inhibitor may be given after adjuvant chemotherapy.
For patients with advanced stage IV TNBC, treatment options can vary based on certain factors. If the patient has a BRCA mutation, the cancer cells may express the PD-L1 protein, which can be detected by an immunohistochemistry assay, or they may also exhibit high microsatellite instability (MSI-H).
In such cases, they might be eligible for alternative treatments. These could include a combination of chemotherapy and immune checkpoint inhibitors, such as Pembrolizumab, or PARP inhibitors like Olaparib or Talzoparib. More recently, antibody drug conjugate therapy has also emerged.
Genialis’ Approach is Different — Enter: RNA Biomarkers
While the biomarkers mentioned above have pointed towards new treatment options for TNBC patients, the biomarker landscape remains fragmented, and the predictive value suboptimal. As for most biomarkers in oncology, DNA or protein are the most commonly measured analyte (e.g. a BRCA mutation or a PD-L1 protein), even though PD-L1 is found in only about 1 out of 5 TNBCs.
Genialis is taking a different approach to solving the problem. RNA is our preferred analyte, because RNA is closer to the phenotype than DNA. While a DNA mutation may be necessary to evaluate a disregulated oncogene, the mutation alone is usually insufficient to predict response to therapy.
In contrast, RNA represents the actuation of biological states, a snapshot of the cellular or tissue physiology. RNA contains high-dimensional information which can be leveraged to understand the biology of a given cancer with far more nuance than DNA alone. Furthermore, Genialis calls coding mutations directly from RNA, so the genomic information is gathered as well.
The XernaTM TME Panel: Unraveling the Tumor Microenvironment
Genialis is the RNA biomarker company and one of the ways we’ve demonstrated our expertise is through development of the Xerna TME Panel. The panel employs machine learning to decipher RNA-sequencing data and dives deep into the intricate biology of the TME.
The panel identifies critical interactions between genes associated with angiogenesis and tumor immune response, to classify tumors into four distinct subtypes:
-
Immune Active (IA),
-
Immune Suppressed (IS),
-
Immune Desert (ID), and
-
Angiogenic (A).
This classification can help predict patient response to various therapies with distinct MOAs, and identify actionable genomic alterations associated with potential targeted therapies.
Investigating Subtypes and Genomic Alterations in TNBC
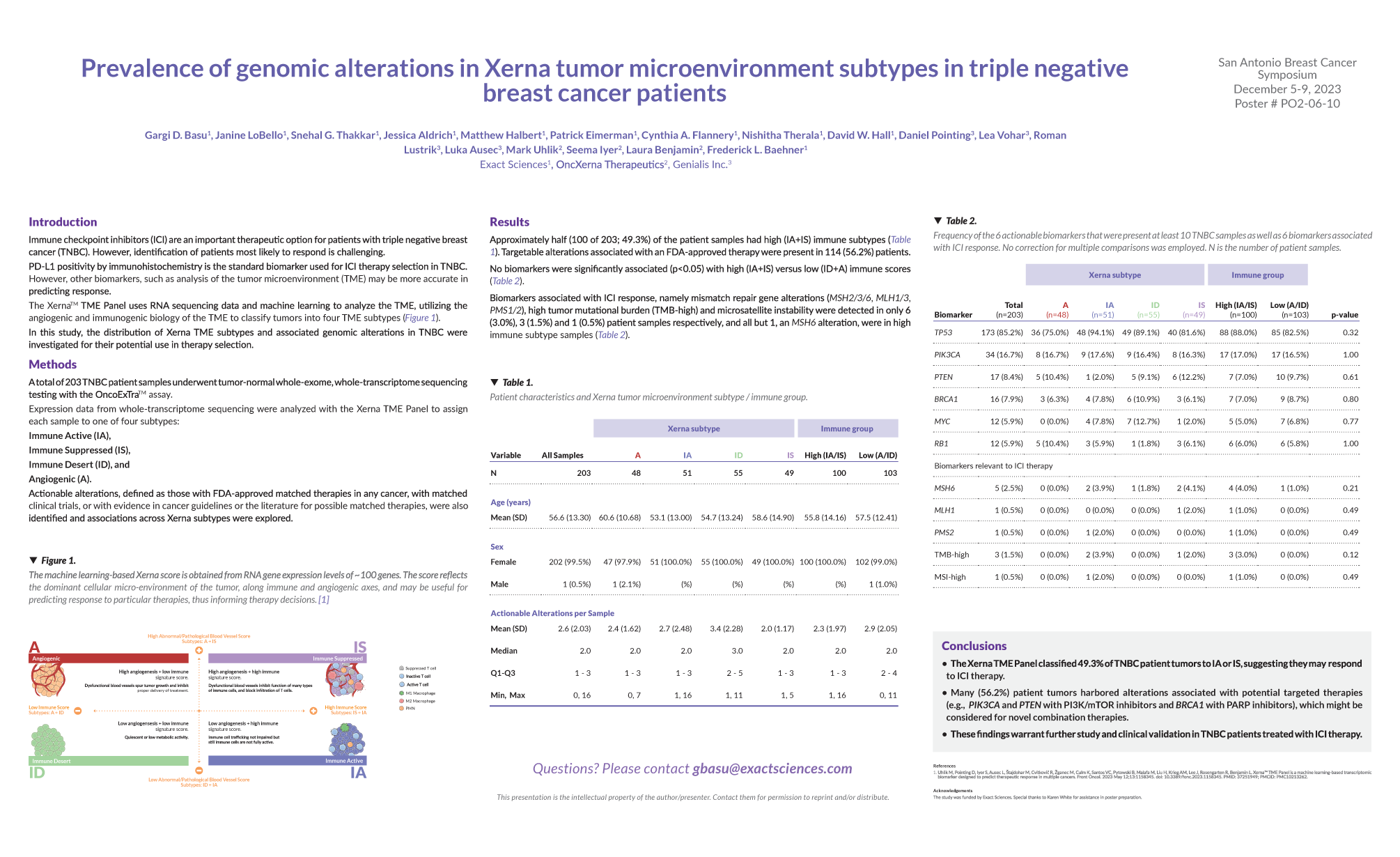
In a recent poster presented by Genialis and Exact Sciences at the San Antonio Breast Cancer Symposium 2023 (SABCS), we explored the prevalence of genomic alterations across the Xerna TME subtypes in 203 TNBC patient samples to shed light on potential therapeutic implications.
Key Findings:
-
Xerna TME Subtypes and ICI Response: The Xerna TME Panel classified 49.3% of TNBC patient tumors as IA or IS, suggesting that these subtypes may respond favorably to ICI therapy.
-
Targeted Therapies: Notably, 56.2% of patient tumors harbored alterations associated with potential targeted therapies. For instance, alterations in genes like PIK3CA and PTEN could be considered for PI3K/mTOR inhibitors, while BRCA1 alterations might guide PARP inhibitor use.
-
Future Directions: These findings warrant further investigation and clinical validation. The Xerna TME Panel holds promise for stratifying TNBC patients and identifying novel combination therapies.
RNA Biomarkers Pave the Way Towards Optimized Breast Cancer Treatment
The SABCS presentation highlights the potential utility of the Xerna TME Panel in personalized treatment decisions. Genialis continues to demonstrate why an analysis of RNA-based biomarkers in the TME may provide multi-dimensional information in predicting response and personalizing therapy versus the standard of care PD-L1 biomarker for ICI therapy.
Looking ahead, we anticipate new advancements in breast cancer management, fueled by the synergy of machine learning and new types of biomarkers. See the poster above and stay connected with Genialis on LinkedIn for future breakthroughs in precision medicine.